Your Epoxide ring opening mechanism images are available in this site. Epoxide ring opening mechanism are a topic that is being searched for and liked by netizens now. You can Find and Download the Epoxide ring opening mechanism files here. Find and Download all free vectors.
If you’re looking for epoxide ring opening mechanism images information linked to the epoxide ring opening mechanism interest, you have visit the ideal site. Our site always provides you with suggestions for seeing the highest quality video and picture content, please kindly hunt and locate more enlightening video articles and graphics that fit your interests.
Epoxide Ring Opening Mechanism. For example treating ethylene oxide with H-X proceeds by the following mechanism. In this video the product of the reaction of cyclohexyl-epoxide with hydrobromic acid HBr is outlined in ACE Organic. DFT calculations indicate the mechanism for isobutylene ring opening involves epoxide activation and ring opening on an alcohol adsorbed onto the catalytic site. The stereoselectivity of these reactions is also like an S N 2 process.
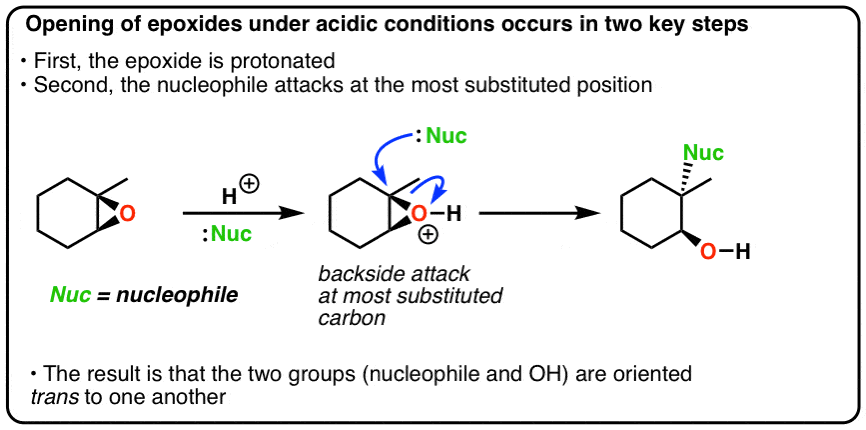 Opening Of Epoxides With Acid Master Organic Chemistry From masterorganicchemistry.com
Opening Of Epoxides With Acid Master Organic Chemistry From masterorganicchemistry.com
Because an epoxide is a type of ether the ring opening of epoxides is an ether cleavage. Step by step electron pushing mechanism Recommend 15x or 2x speed. The transition state has more progress toward the C-LG bond breaking than an S N 2 but more progress toward the C-Nu bond forming than S N 1. First the oxygen is protonated creating a good leaving group step 1 below. DFT calculations indicate the mechanism for isobutylene ring opening involves epoxide activation and ring opening on an alcohol adsorbed onto the catalytic site. The acidolysis ring-opening reaction refers to the nucleophilic addition reaction of epoxides with acids to prepare hydroxyalkyl esters.
The epoxide ring is opened by an S N 2 like mechanism so the two -OH groups will be trans to each other in the product.
Probably the best way to depict the acid-catalyzed epoxide ring-opening reaction is as a hybrid or cross between an S N 2 and S N 1 mechanism. The phenol-epoxide ring-opening reaction is performed through such a reaction mechanism. Probably the best way to depict the acid-catalyzed epoxide ring-opening reaction is as a hybrid or cross between an S N 2 and S N 1 mechanism. DFT calculations indicate the mechanism for isobutylene ring opening involves epoxide activation and ring opening on an alcohol adsorbed onto the catalytic site. For epichlorohydrin the activation energy barrier and experimentally observed regioselectivity are found using DFT to be consistent with a concerted reaction mechanism involving activation of. One advantage is that ether linkages.
 Source: researchgate.net
Source: researchgate.net
Although the coupling reaction of the GMA with macromolecules has widely been investigated there are still mechanisms that remain to be explained when GMA is processed in an aqueous solution at. The first step of the mechanism is an acid-base reaction. In the present study we have discovered reversible epoxide openingclosing reactions in GO upon alkaline and acid treatments respectively under ambient conditions. Epoxides how-ever are opened readily by basic reagents. Because an epoxide is a type of ether the ring opening of epoxides is an ether cleavage.
 Source: researchgate.net
Source: researchgate.net
Base-Catalyzed Ring-Opening of Epoxides Due to their. Probably the best way to depict the acid-catalyzed epoxide ring-opening reaction is as a hybrid or cross between an S N 2 and S N 1 mechanism. Base-catalyzed ring-openings of epoxides show typical S N 2-like regioselectivity in which the nucleophile attacks the less hindered carbon. The acidolysis ring-opening reaction refers to the nucleophilic addition reaction of epoxides with acids to prepare hydroxyalkyl esters. And in the second step we add a proton source.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
When polymerization does occur if the rate of epoxide ring-opening is faster than the rate of CO 2 insertion the resulting polycarbonate can contain a mixture of both ether and carbonate linkages. Step by step electron pushing mechanism Recommend 15x or 2x speed. DFT calculations indicate the mechanism for isobutylene ring opening involves epoxide activation and ring opening on an alcohol adsorbed onto the catalytic site. When polymerization does occur if the rate of epoxide ring-opening is faster than the rate of CO 2 insertion the resulting polycarbonate can contain a mixture of both ether and carbonate linkages. The acidolysis ring-opening reaction refers to the nucleophilic addition reaction of epoxides with acids to prepare hydroxyalkyl esters.
 Source: youtube.com
Source: youtube.com
Epoxides how-ever are opened readily by basic reagents. So when I draw the product of. Probably the best way to depict the acid-catalyzed epoxide ring-opening reaction is as a hybrid or cross between an S N 2 and S N 1 mechanism. Is epoxide ring opening reversible. Lone pair of electrons on oxygen are going to pick up that proton.
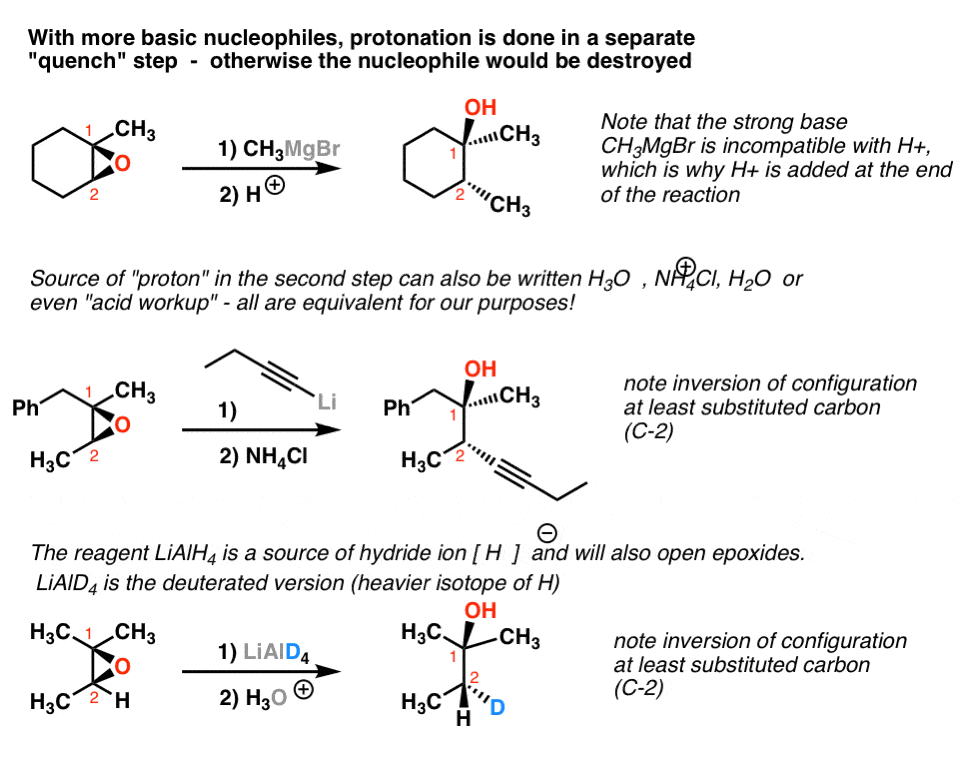 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The amount of ether linkages in the copolymer can be tuned. This is an acid-catalyzed reaction so theyre H plus protons floating around. If the carbons of the epoxide are 1 or 2 then the epoxide opening goes predominantly by an S N2 mechanism and the nucleophile adds to the least substituted carbon if either carbon of the epoxide is 3 the epoxide opening goes predominantly by an S. Kontaktieren Sie uns einfach für eine schnelle Hilfe. Regiochemistry of acid-catalyzed epoxide openings.
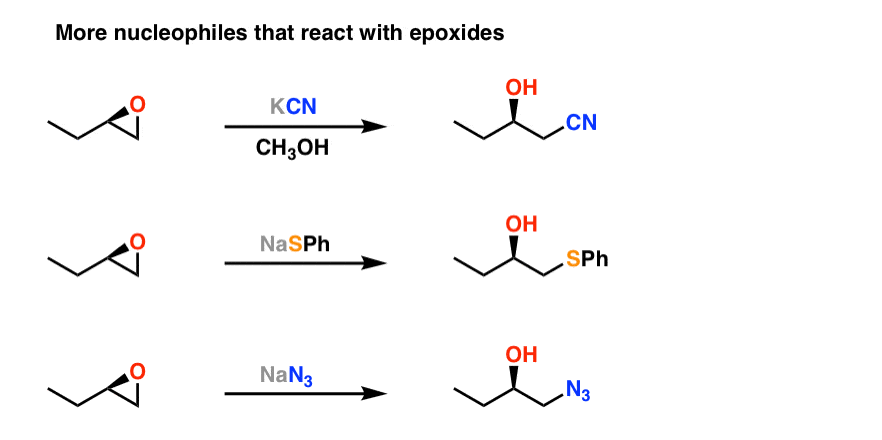 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Lone pair of electrons on oxygen are going to pick up that proton. Is epoxide ring opening reversible. The acid-catalyzed epoxide ring-opening reaction mechanism is analogous to the formation of the bromonium ion in halogenation of alkenes and mercurium ion formation in oxymercurationdemercuratioin or alkoxymercurationdemercuration. In the present study we have discovered reversible epoxide openingclosing reactions in GO upon alkaline and acid treatments respectively under ambient conditions. So in this video well look at the ring opening reactions of epoxides using strong nucleophiles.
 Source: chemistry-europe.onlinelibrary.wiley.com
Source: chemistry-europe.onlinelibrary.wiley.com
So in this video well look at the ring opening reactions of epoxides using strong nucleophiles. The amount of ether linkages in the copolymer can be tuned. First the oxygen is protonated creating a good leaving group step 1 below. So in the first step we add a strong nucleophile to our epoxide. Epoxides how-ever are opened readily by basic reagents.
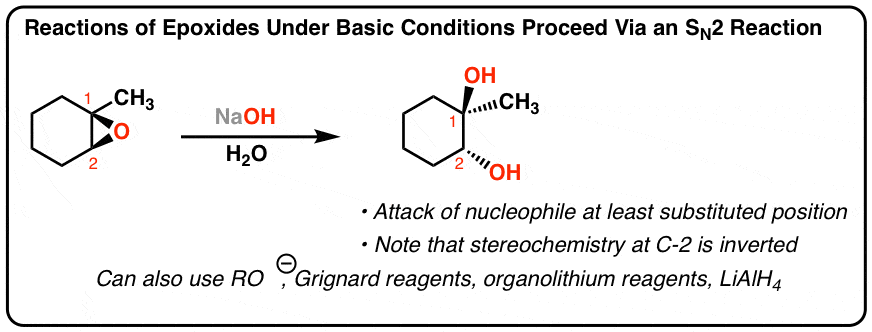 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Epoxide ring opening of epichlorohydrin with methanol than Al-Beta. If the carbons of the epoxide are 1 or 2 then the epoxide opening goes predominantly by an S N2 mechanism and the nucleophile adds to the least substituted carbon if either carbon of the epoxide is 3 the epoxide opening goes predominantly by an S. Although the acid-catalyzed ring-opening of epoxides follows a mechanism with S N 2 features inversion of stereochemistry no carbocation rearrangements the mechanism is not strictly a S N 2 mechanism. Acid Catalyzed Ring Opening In acid catalyzed ring-opening the epoxide oxygen is protonated under acidic conditions activating it for nucleophilic attack. In this video the product of the reaction of cyclohexyl-epoxide with hydrobromic acid HBr is outlined in ACE Organic.
 Source: chemistrysteps.com
Source: chemistrysteps.com
And in the second step we add a proton source. Transesterification and epoxide ring-opening reactions are two mechanism routes that explain chemical modifications of macromolecules by glycidyl methacrylate GMA. For epichlorohydrin the activation energy barrier and experimentally observed regioselectivity are found using DFT to be consistent with a concerted reaction mechanism involving activation of. The acid catalyst converts the epoxide oxygen a poor leaving group into an oxonium ion a better leaving group making the reaction feasible. Probably the best way to depict the acid-catalyzed epoxide ring-opening reaction is as a hybrid or cross between an S N 2 and S N 1 mechanism.
 Source: chemistrysteps.com
Source: chemistrysteps.com
First the oxygen is protonated creating a good leaving group step 1 below. This ring strain acts as a driving force for epoxides to undergo ring-opening reactions either with halogen acids or weak nucleophiles in the presence of mild acid. The epoxide ring is opened by an S N 2 like mechanism so the two -OH groups will be trans to each other in the product. The transition state has more progress toward the C-LG bond breaking than an S N 2 but more progress toward the C-Nu bond forming than S N 1. For epichlorohydrin the activation energy barrier and experimentally observed regioselectivity are found using DFT to be consistent with a concerted reaction mechanism involving activation of.
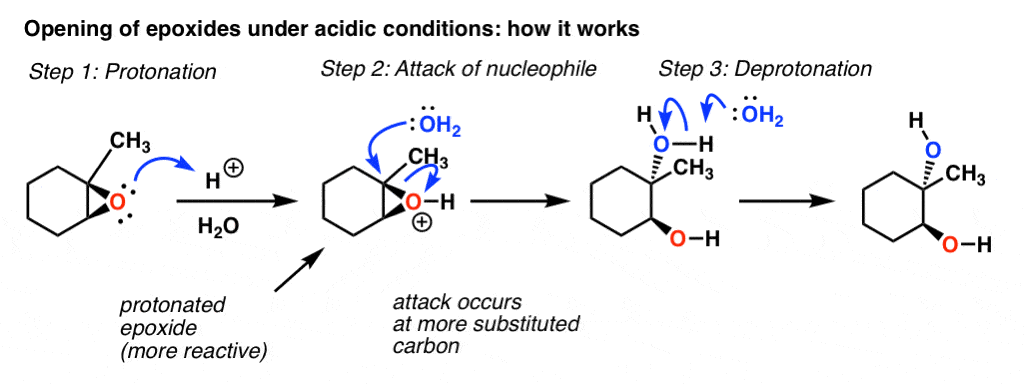 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The acidolysis ring-opening reaction refers to the nucleophilic addition reaction of epoxides with acids to prepare hydroxyalkyl esters. Epoxides how-ever are opened readily by basic reagents. Epoxides are more reactive than acycl. Lone pair of electrons on oxygen are going to pick up that proton. One advantage is that ether linkages.
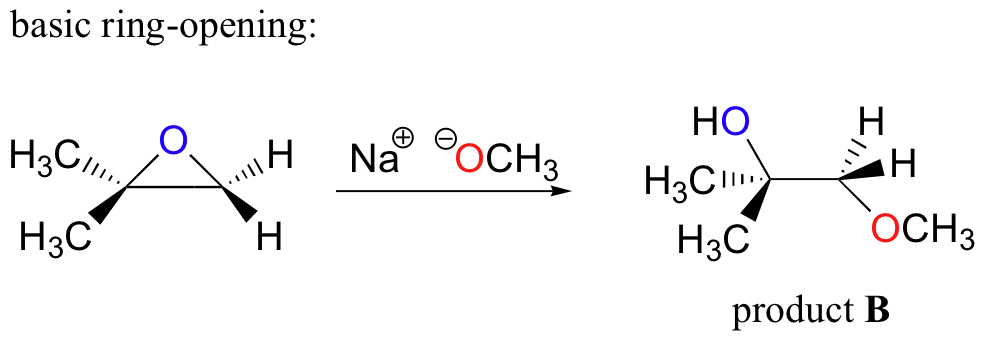 Source: chem.libretexts.org
Source: chem.libretexts.org
Probably the best way to depict the acid-catalyzed epoxide ring-opening reaction is as a hybrid or cross between an S N 2 and S N 1 mechanism. For Sn-Beta the activation energy for the reaction between epichlorohydrin and methanol is determined to be 537 kJ mol-1. The important functions of the imidazole catalyst are to reduce the ΔG value of the phenol-epoxide ring-opening reaction by about 20 kcalmol or more and to generate a more nucleophilic phenoxide ion as the reaction proceeds. They undergo ring-opening reactions under acidic or basicnucleophilic conditions. Regiochemistry of acid-catalyzed epoxide openings.
 Source: polymerdatabase.com
Source: polymerdatabase.com
Epoxide Ring-Opening with Strong Nucleophiles The ring-opening reactions of epoxides occur via SN2 mechanism where the oxygen of the epoxide is the leaving group. The amount of ether linkages in the copolymer can be tuned. The transition state has more progress toward the C-LG bond breaking than an S N 2 but more progress toward the C-Nu bond forming than S N 1. The important functions of the imidazole catalyst are to reduce the ΔG value of the phenol-epoxide ring-opening reaction by about 20 kcalmol or more and to generate a more nucleophilic phenoxide ion as the reaction proceeds. And in the second step we add a proton source.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The epoxide ring is opened by an S N 2 like mechanism so the two -OH groups will be trans to each other in the product. The first step of the mechanism is an acid-base reaction. Because an epoxide is a type of ether the ring opening of epoxides is an ether cleavage. First the oxygen is protonated creating a good leaving group step 1 below. One advantage is that ether linkages.
 Source: youtube.com
Source: youtube.com
The acid-catalyzed epoxide ring-opening reaction mechanism is analogous to the formation of the bromonium ion in halogenation of alkenes and mercurium ion formation in oxymercurationdemercuratioin or alkoxymercurationdemercuration. When polymerization does occur if the rate of epoxide ring-opening is faster than the rate of CO 2 insertion the resulting polycarbonate can contain a mixture of both ether and carbonate linkages. First the oxygen is protonated creating a good leaving group step 1 below. However the reaction of chondroitin sulfate by GMA at pH 3 indeed occurs via epoxide ring opening13 In this case there is an attachment of a whole GMA molecule onto the sulfate and carboxylic groups of the chondroitin sulfate. Step by step electron pushing mechanism Recommend 15x or 2x speed.
 Source: polymerdatabase.com
Source: polymerdatabase.com
Re-call that ordinary ethers do not undergo cleavage in base Eq. Because an epoxide is a type of ether the ring opening of epoxides is an ether cleavage. They undergo ring-opening reactions under acidic or basicnucleophilic conditions. In the present study we have discovered reversible epoxide openingclosing reactions in GO upon alkaline and acid treatments respectively under ambient conditions. First the oxygen is protonated creating a good leaving group step 1 below.
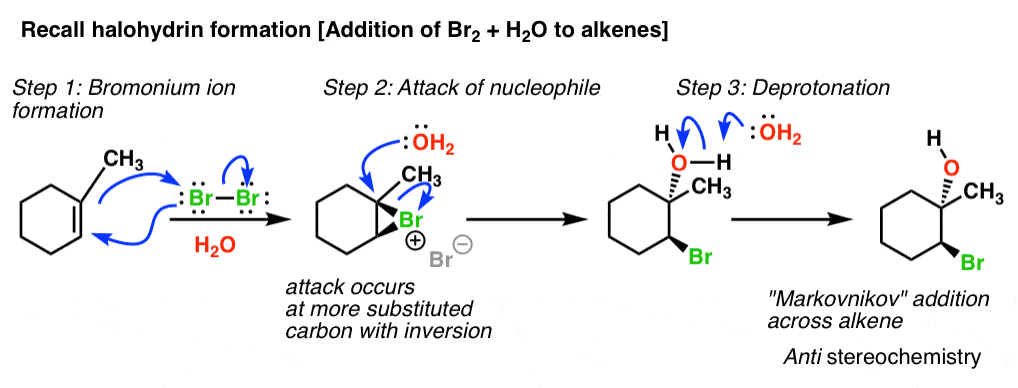 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Although the acid-catalyzed ring-opening of epoxides follows a mechanism with S N 2 features inversion of stereochemistry no carbocation rearrangements the mechanism is not strictly a S N 2 mechanism. Ad Sie brauchen eine neue Bodenbeschichtung. Epoxides are more reactive than acycl. The epoxide ring is opened by an S N 2 like mechanism so the two -OH groups will be trans to each other in the product. The acidolysis ring-opening reaction refers to the nucleophilic addition reaction of epoxides with acids to prepare hydroxyalkyl esters.
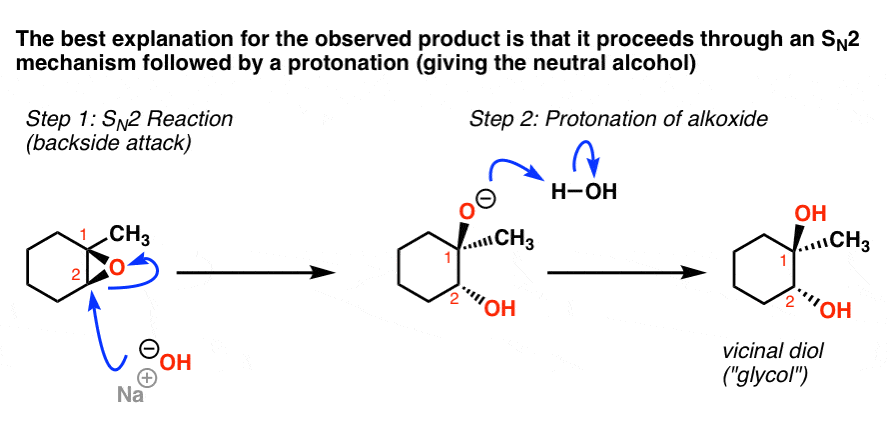 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
General Reaction Mechanism Basic Hydrolysis Under aqueous basic conditions the epoxide is opened by the attack of hydroxide nucleophile during an S N 2 reaction. This ring strain acts as a driving force for epoxides to undergo ring-opening reactions either with halogen acids or weak nucleophiles in the presence of mild acid. Epoxides are more reactive than acycl. Probably the best way to depict the acid-catalyzed epoxide ring-opening reaction is as a hybrid or cross between an S N 2 and S N 1 mechanism. Is epoxide ring opening reversible.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title epoxide ring opening mechanism by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






