Your Cyclohexane rings attached images are ready in this website. Cyclohexane rings attached are a topic that is being searched for and liked by netizens now. You can Download the Cyclohexane rings attached files here. Get all free photos.
If you’re looking for cyclohexane rings attached pictures information linked to the cyclohexane rings attached keyword, you have pay a visit to the ideal blog. Our site always gives you suggestions for seeing the highest quality video and image content, please kindly hunt and locate more enlightening video content and images that fit your interests.
Cyclohexane Rings Attached. It can be seen. Rather if you have a cyclic substituent like cyclohexane or cyclopropane you would treat it using substituent naming adding a -yl. In 154 and 155 the orientations of the C 25 and C 24 methyl groups were reversed as compared to 152 and 153. The key difference between cis cyclohexane and trans cyclohexane is that cis cyclohexane has its substituents pointing to the same plane of the ring whereas trans cyclohexane has its.
 Cycloalkanes Introduction To Chemistry From courses.lumenlearning.com
Cycloalkanes Introduction To Chemistry From courses.lumenlearning.com
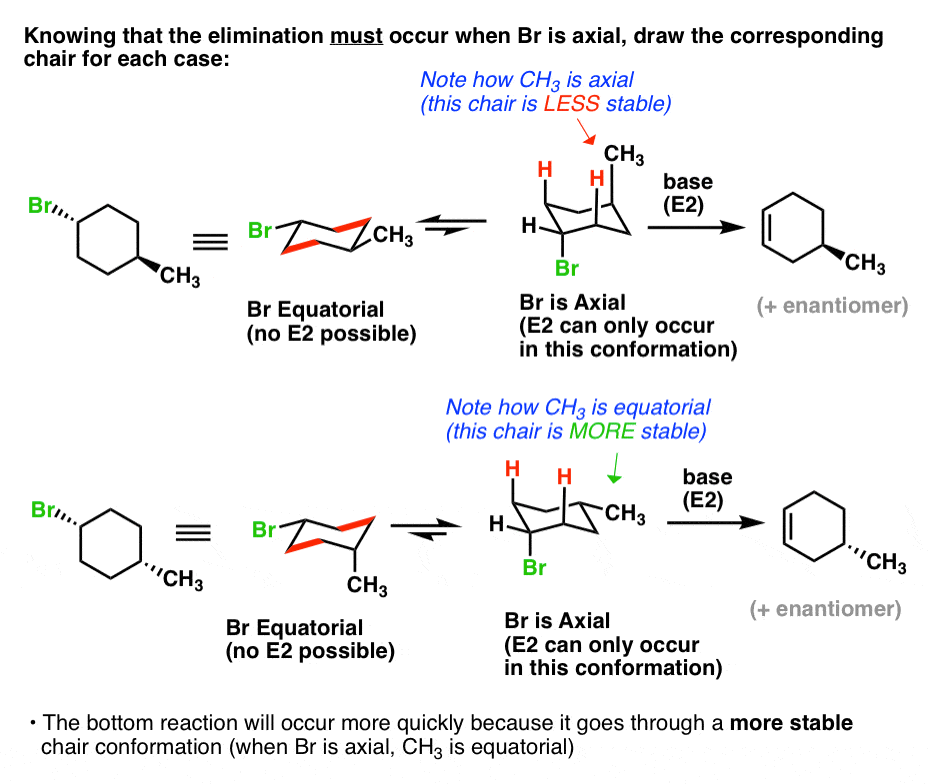
Suppose we are asked to determine which compound is expected to react faster in E2 elimination. When more than one similar substituent group are present in the ring they are labeled with the Greek numerical prefixes such as di tri tetra to denote the number of similar substituent groups attached to the ring. We write it as C-2 CHH with the elements in parentheses listed in order of decreasing atomic number. And this is a true or false question regarding whether the structure shown is a cyclohexane ri 1. The key difference between cis cyclohexane and trans cyclohexane is that cis cyclohexane has its substituents pointing to the same plane of the ring whereas trans cyclohexane has its. The cyclic form of hexane used as a raw material in the manufacture of nylon.
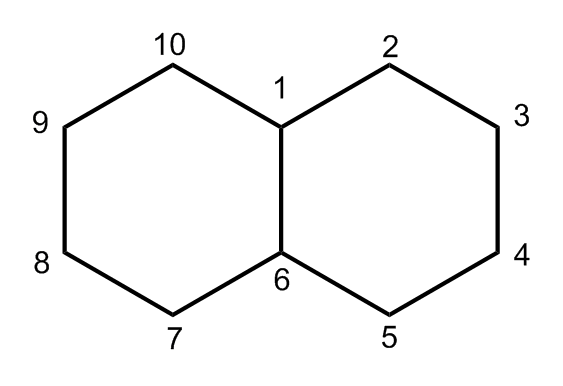
After assigning carbon 1 the cyclohexane ring can be numbered going clockwise or counterclockwise.
We wont be expected to use phenyl or benzyl. Suppose we are asked to determine which compound is expected to react faster in E2 elimination. The cyclic form of hexane used as a raw material in the manufacture of nylon. Vitamin A beta-carotene beta-ionone. In 152 and 153 the C 25 and C 24 methyl groups occupy axial and equatorial orientations respectively. Cycloalkanesis the smallest cycloalkane whereas cyclohexane C6H12 is the most studied best understood and most important.
Source: quora.com
Except for the natural form the ether oxygen at C 7 was attached axially to the cyclohexanone ring. Which substituted cyclohexane is correctly named as a cyclohexyl alkane. The ethyl group attachment is assigned carbon 1 because ethyl comes before methyl alphabetically. We write it as C-5 CHH So C-2 and C-6 are still the same. Cyclohexanone has one ketone functional group which is attached with a cyclic six carbon ring.
 Source: chem.libretexts.org
Source: chem.libretexts.org
The ethyl group attachment is assigned carbon 1 because ethyl comes before methyl alphabetically. Therefore the preferred IUPAC name for the compound that is given in the question is formed according to Rule 1 as 44-dimethyl-11-bi cyclohexane. C07C40300 Derivatives of cyclohexane or of a cyclohexene or of cyclohexadiene having a side-chain containing an acyclic unsaturated part of at least four carbon atoms this part being directly attached to the cyclohexane or cyclohexene or cyclohexadiene rings eg. If two bromo- groups are attached to the adjacent carbon atoms of the benzene ring it is named as 12-dibromobenzene. Answered The following structure shows a cyclohexane ring with two attached methyl groups.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
When more than one similar substituent group are present in the ring they are labeled with the Greek numerical prefixes such as di tri tetra to denote the number of similar substituent groups attached to the ring. We write it as C-5 CHH So C-2 and C-6 are still the same. How fast a given cyclohexane undergoes E2 elimination depends on how stable the chair conformation with the leaving group in the axial position is. Therefore the preferred IUPAC name for the compound that is given in the question is formed according to Rule 1 as 44-dimethyl-11-bi cyclohexane. Cheerleader3056 01092018 Chemistry High School 5 pts.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
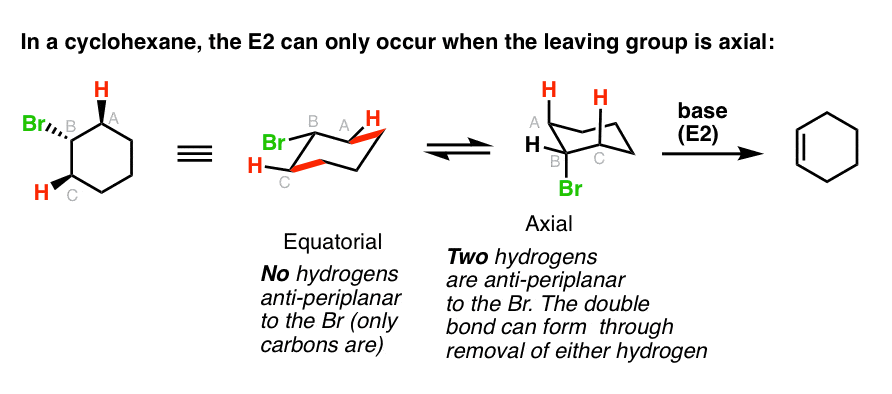
After assigning carbon 1 the cyclohexane ring can be numbered going clockwise or counterclockwise. Which substituted cyclohexane is correctly named as a cyclohexyl alkane. Bring-carbon atoms are sp hybridized and the methyl group is sp hybridized. An atom or groups of atoms that substitute for a hydrogen atom in an organic compound giving the compound unique chemical properties and determining its reactivity. How fast a given cyclohexane undergoes E2 elimination depends on how stable the chair conformation with the leaving group in the axial position is.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
And this is a true or false question regarding whether the structure shown is a cyclohexane ri 1. The main difference between compound 1b and compound 1c is the position of the carbonyl group on the 2-pyrrolidine ring with respect to the methyl group on the. Cyclohexanol is a cyclohexane with an alcohol group directly attached to the ring. All bond angles are. Six of the positions exist in vertical positions known as axial positions and the other six point out and away from the center of the ring.
 Source: thechemistryguru.com
Source: thechemistryguru.com
All bond angles are. Vitamin A beta-carotene beta-ionone. Cycloalkanesis the smallest cycloalkane whereas cyclohexane C6H12 is the most studied best understood and most important. We write it as C-5 CHH So C-2 and C-6 are still the same. C-6 is attached to C-5 H and H.
 Source: sciencedirect.com
Source: sciencedirect.com
We write it as C-2 CHH with the elements in parentheses listed in order of decreasing atomic number. C07c40316derivatives of cyclohexane or of a cyclohexene or of cyclohexadiene having a side-chain containing an acyclic unsaturated part of at least. We write it as C-2 CHH with the elements in parentheses listed in order of decreasing atomic number. Cyclohexane six-carbon ring or cyclooctane eight-carbon ring 2 Number the ring starting from the carbon that will give the lowest. Rather if you have a cyclic substituent like cyclohexane or cyclopropane you would treat it using substituent naming adding a -yl.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
For example lets compare these two compounds. Which substituted cyclohexane is correctly named as a cyclohexyl alkane. With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of the cycloalkanes. A ket one is possible but we call that structure cyclohexan one. The bicyclic skeleton consists of a lactam ring attached by a spiro junction to a cyclohexane ring.
 Source: researchgate.net
Source: researchgate.net
There are twelve possible positions on a chair structure. With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of the cycloalkanes. Rather difficult to add an aldehyde in the ring. Six of the positions exist in vertical positions known as axial positions and the other six point out and away from the center of the ring. The main difference between compound 1b and compound 1c is the position of the carbonyl group on the 2-pyrrolidine ring with respect to the methyl group on the.
 Source: sciencedirect.com
Source: sciencedirect.com
Since the early work by Lemieux Kullnig Bernstein and Schneider 1539 many attempts have been made to correlate the chemical shifts of protons in the functional groups attached to six-membered rings principally cyclohexane and pyranose monosaccharides with their configuration and a number of such correlations are summarized in Table 3-8-9. Other articles where cyclohexane is discussed. Locants including the locants 1 and 1 are necessary in preferred IUPAC names to indicate points of attachment of rings or ring systems see P-2821. After assigning carbon 1 the cyclohexane ring can be numbered going clockwise or counterclockwise. Bring-carbon atoms are sp hybridized and the methyl group is sp hybridized.
 Source: sciencedirect.com
Source: sciencedirect.com
With no torsional strain and no angle strain cyclohexane is the most stable of all the small rings of the cycloalkanes. Other articles where cyclohexane is discussed. The lactam ring adopts an envelope conformation and the cyclohexane ring has a chair conformation. Vitamin A beta-carotene beta-ionone. It can be seen.
 Source: sciencedirect.com
Source: sciencedirect.com
C-6 is attached to C-5 H and H. Answered The following structure shows a cyclohexane ring with two attached methyl groups. Cyclohexane is an alicyclic hydrocarbon comprising a ring of six carbon atoms. Rather if you have a cyclic substituent like cyclohexane or cyclopropane you would treat it using substituent naming adding a -yl. Locants including the locants 1 and 1 are necessary in preferred IUPAC names to indicate points of attachment of rings or ring systems see P-2821.
 Source: chem.libretexts.org
Source: chem.libretexts.org
C07C40300 Derivatives of cyclohexane or of a cyclohexene or of cyclohexadiene having a side-chain containing an acyclic unsaturated part of at least four carbon atoms this part being directly attached to the cyclohexane or cyclohexene or cyclohexadiene rings eg. We write it as C-2 CHH with the elements in parentheses listed in order of decreasing atomic number. Six of the positions exist in vertical positions known as axial positions and the other six point out and away from the center of the ring. Cyclohexanol is a cyclohexane with an alcohol group directly attached to the ring. For example a 7 long carbon chain with a cyclopropane attached to the third carbon would be called 3-cyclopropylheptane.
 Source: sciencedirect.com
Source: sciencedirect.com
All bond angles are. C-2 is attached to C-3 H and H. The three dimensional shapes assumed by the common rings especially cyclohexane and larger rings are described and discussed in the Conformational Analysis Section. Hydrocarbons having more than one ring are common and are referred to as bicyclic two rings tricyclic three rings and in general polycyclic compounds. All carbon atoms are sp hybridized.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Cycloalkanes are alkanes with carbon atoms attached in the form of a closed ring. In 152 and 153 the C 25 and C 24 methyl groups occupy axial and equatorial orientations respectively. If two bromo- groups are attached to the adjacent carbon atoms of the benzene ring it is named as 12-dibromobenzene. For example a 7 long carbon chain with a cyclopropane attached to the third carbon would be called 3-cyclopropylheptane. C07c40316derivatives of cyclohexane or of a cyclohexene or of cyclohexadiene having a side-chain containing an acyclic unsaturated part of at least.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
In 154 and 155 the orientations of the C 25 and C 24 methyl groups were reversed as compared to 152 and 153. It can be seen. From the IR spectrum of Cyclohexanone a sharp stretch displays at around. After assigning carbon 1 the cyclohexane ring can be numbered going clockwise or counterclockwise. The three dimensional shapes assumed by the common rings especially cyclohexane and larger rings are described and discussed in the Conformational Analysis Section.
Source: quora.com
Granted the base and leaving group are the same the rate of the reaction will. Therefore the preferred IUPAC name for the compound that is given in the question is formed according to Rule 1 as 44-dimethyl-11-bi cyclohexane. After assigning carbon 1 the cyclohexane ring can be numbered going clockwise or counterclockwise. Suppose we are asked to determine which compound is expected to react faster in E2 elimination. If two bromo- groups are attached to the adjacent carbon atoms of the benzene ring it is named as 12-dibromobenzene.
 Source: slideplayer.com
Source: slideplayer.com
After assigning carbon 1 the cyclohexane ring can be numbered going clockwise or counterclockwise. C07c40316derivatives of cyclohexane or of a cyclohexene or of cyclohexadiene having a side-chain containing an acyclic unsaturated part of at least. The ring is named by the number of carbons as before but with the prefix cyclo-. The ending al is used for an aldehyde a CHO group. If two bromo- groups are attached to the adjacent carbon atoms of the benzene ring it is named as 12-dibromobenzene.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title cyclohexane rings attached by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






