Your Benzene ring ir spectrum images are ready. Benzene ring ir spectrum are a topic that is being searched for and liked by netizens today. You can Download the Benzene ring ir spectrum files here. Download all free photos.
If you’re looking for benzene ring ir spectrum pictures information connected with to the benzene ring ir spectrum keyword, you have pay a visit to the ideal blog. Our website always gives you suggestions for refferencing the maximum quality video and image content, please kindly surf and find more enlightening video articles and images that fit your interests.
Benzene Ring Ir Spectrum. Infrared Spectrum of Toluene Ultraviolet Spectroscopy The presence of conjugated π bonds makes aromatic rings detectable in a UVVis spectrum. Pics of. If you are talking about the experimental identification of a benzene ring I would use a combination of infrared and NMR spectroscopy. The three possible isomers are easy to differen ate by IR.
 Image Diagram Infrared Spectrum Of Benzene Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Benzene Doc Brown S Advanced Organic Chemistry Revision Notes From docbrown.info
Image Diagram Infrared Spectrum Of Benzene Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Benzene Doc Brown S Advanced Organic Chemistry Revision Notes From docbrown.info
The position of substitution on a benzene ring can sometimes be determined from the IR spectrum. Ir Spectrum Table Benzene Ring. If you are talking about the experimental identification of a benzene ring I would use a combination of infrared and NMR spectroscopy. The three possible isomers are easy to differen ate by IR. The 3000 cm -1 separation between sp 2 and sp 3 C-H stretching modes is clearly evident. NIST Chemistry WebBook The National Institute of Standards and Technology NIST uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment.
Do Halogens Appear In The Ir Spectrum For Example A Molecule.
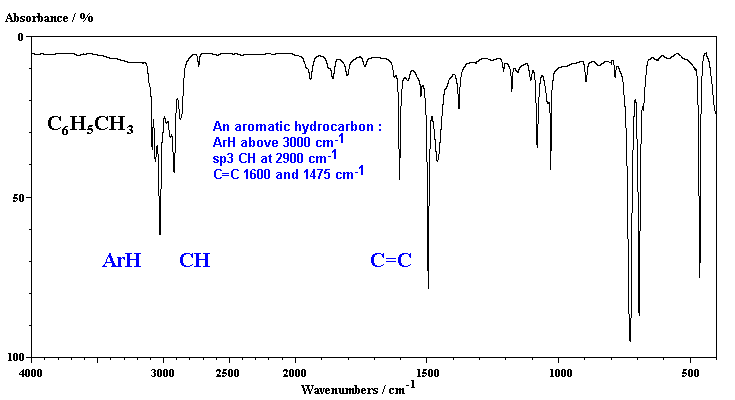
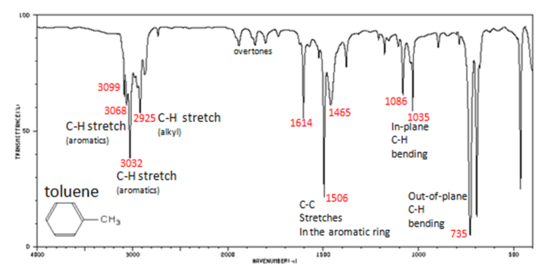
Then we examine in detail the spectra of mono- and di-substituted benzene rings and learn that infrared spectroscopy easily distinguishes between ortho- meta- and para. CH stretch from 3100-3000 cm -1 overtones weak from 2000-1665 cm -1 CC stretch in-ring from 1600-1585 cm -1 CC stretch in-ring from 1500-1400 cm -1 CH oop from 900-675 cm -1 The spectrum of toluene is shown below. Do Halogens Appear In The Ir Spectrum For Example A Molecule. 93 rows In physical and analytical chemistry infrared spectroscopy IR spectroscopy is a. Infrared Spectrum of Toluene Ultraviolet Spectroscopy The presence of conjugated π bonds makes aromatic rings detectable in a UVVis spectrum. Ir Spectrum Table Benzene Ring.
 Source: docbrown.info
Source: docbrown.info
The full IR spectra are shown on this page with an. Benzene primarily absorbs through a be a π-π transition over the range from 160-208 nm wi th a λ max v alue of about 178 nm. Do Halogens Appear In The Ir Spectrum For Example A Molecule. Also benzene rings show overtones in the 1500-1700 cm-1region even though these arise from complex ring deformations. Some of the characteristic absorptions are mνcm-1mllVibration mode.
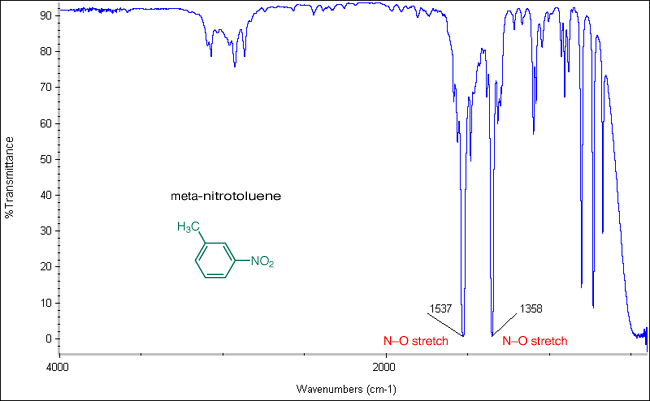 Source: orgchemboulder.com
Source: orgchemboulder.com
Also benzene rings show overtones in the 1500-1700 cm-1region even though these arise from complex ring deformations. Infrared Spectrum of Toluene Ultraviolet Spectroscopy The presence of conjugated π bonds makes aromatic rings detectable in a UVVis spectrum. This note demonstrates one specific case how two sets of IR bands show the rela ve posi ons of the subs tuents on a disubs tuted benzene ring. Together creating precise yet practical optical solutions enabling you to solve problems. Some of the characteristic absorptions are mνcm-1mllVibration mode.
Source: webbook.nist.gov
Has CO band 1650-1800 cm-1 very strong does not have CO band IR Spectrum aldehydes C O aldehyde C-H 1725-1740 saturated 1660-1700 unsaturated 2860-2800 2760-2700 both. Continuing our theme of investigating the infrared spectra of hydrocarbons we look at the nature of aromatic bonding and why aromatic rings have unique structures bonding and infrared spectra. Infrared Spectrum of Toluene Ultraviolet Spectroscopy The presence of conjugated π bonds makes aromatic rings detectable in a UVVis spectrum. Benzene primarily absorbs through a be a π-π transition over the range from 160-208 nm wi th a λ max v alue of about 178 nm. Substitution sensitive bands of benzene below 1000 cm 1.
 Source: docbrown.info
Source: docbrown.info
Ir Spectrum Table Benzene Ring Major Absorptions In Ir Spectra Of Bsmps Diffe Sizes See also Detroit Tigers Seat Chart Quantitative Comonomer Analysis Of Polyacrylates Via Ir Solved Predict The Ir Spectra Of Following Molecules Infrared Spectroscopy See also Time Warner Cable Arena Seating Chart For Concerts. Substitution Pattern Appearance Position of Absorption cm-1 monosubstituted two peaks 730-770 690-710 o-disubstituted. Continuing our theme of investigating the infrared spectra of hydrocarbons we look at the nature of aromatic bonding and why aromatic rings have unique structures bonding and infrared spectra. In the spectrum of benzene this peak falls at 674 cm-1 because the molecule is unsubstituted. The 3000 cm -1 separation between sp 2 and sp 3 C-H stretching modes is clearly evident.
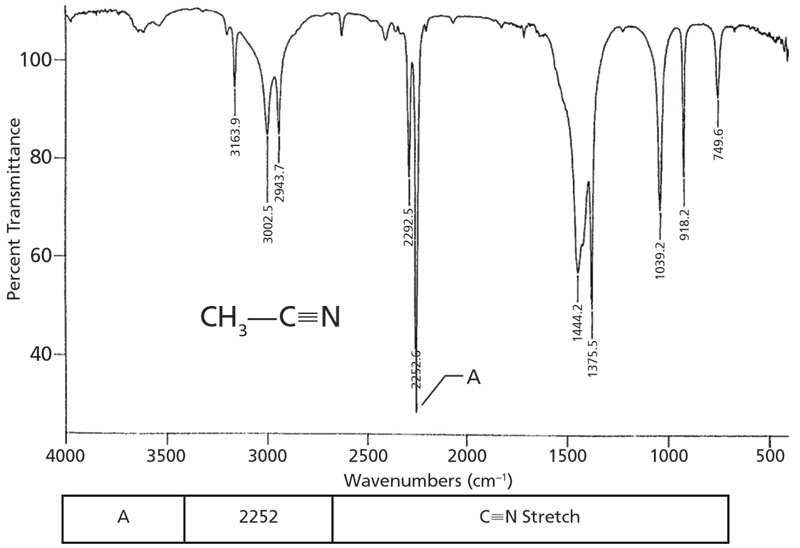 Source: spectroscopyonline.com
Source: spectroscopyonline.com
SOLUTION 10 IN CCl4 FOR 3800-1300 10 IN CS2 FOR 1300-625 AND 10 IN CCl4 FOR 625-240 CM-1 VS. Bands above 730 cm 1 are from out-of-plane CCH bending CH wag modes while those below are from ring bending. Infrared Spectrum of Toluene Ultraviolet Spectroscopy The presence of conjugated π bonds makes aromatic rings detectable in a UVVis spectrum. The patterns observed are summarized in the following table. Ir Aromatics Major Absorptions In Ir Spectra Of Bsmps Diffe Sizes Spectral Ft Ir Data Of Synthesized Compounds Table Infrared Spectrometry Infrared Spectral Interpretation The Benzene Fingers Part I Overtone And Combination Bands See.
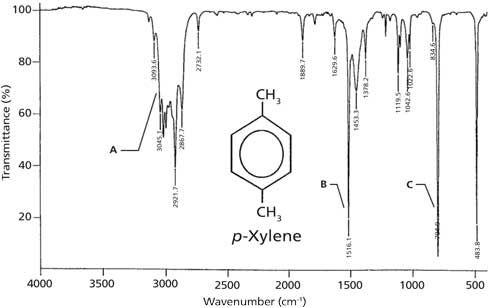 Source: spectroscopyonline.com
Source: spectroscopyonline.com
Benzene shows a less intense absorption in 230-276 nm range. Has CO band 1650-1800 cm-1 very strong does not have CO band IR Spectrum aldehydes C O aldehyde C-H 1725-1740 saturated 1660-1700 unsaturated 2860-2800 2760-2700 both. Carbonyl - compounds For simple aldehydes and ketones the stretching vibration of the carbonyl group has a strong infrared absorption between 1710 and 1740 cm-1. SOLUTION 10 IN CCl4 FOR 3800-1300 10 IN CS2 FOR 1300-625 AND 10 IN CCl4 FOR 625-240 CM-1 VS. The patterns observed are summarized in the following table.
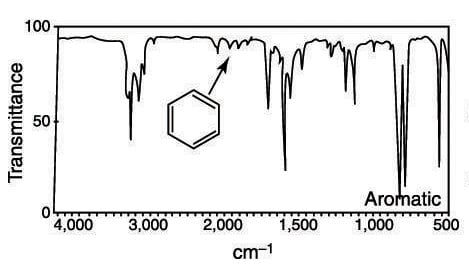 Source: dummies.com
Source: dummies.com
Then we examine in detail the spectra of mono- and di-substituted benzene rings and learn that infrared spectroscopy easily distinguishes between ortho- meta- and para. GAS 70 mmHg N2 ADDED TOTAL PRESSURE 600 mmHg. If you are talking about the experimental identification of a benzene ring I would use a combination of infrared and NMR spectroscopy. The patterns observed are summarized in the following table. In the spectrum of benzene this peak falls at 674 cm-1 because the molecule is unsubstituted.
 Source: chem.ucalgary.ca
Source: chem.ucalgary.ca
The normal way to approach interpretation of an IR spectrum is to examine the functional group region to determine which groups might be present then to. Ir Spectrum Table Benzene Ring Major Absorptions In Ir Spectra Of Bsmps Diffe Sizes See also Detroit Tigers Seat Chart Quantitative Comonomer Analysis Of Polyacrylates Via Ir Solved Predict The Ir Spectra Of Following Molecules Infrared Spectroscopy See also Time Warner Cable Arena Seating Chart For Concerts. Substitution Pattern Appearance Position of Absorption cm-1 monosubstituted two peaks 730-770 690-710 o-disubstituted. Bands above 730 cm 1 are from out-of-plane CCH bending CH wag modes while those below are from ring bending. Ir Spectrum Table Benzene Ring.
 Source: docbrown.info
Source: docbrown.info
Benzene rings often give characteristic absorptions at about 680-900 cm-1. Benzene rings often give characteristic absorptions at about 680-900 cm-1. The full IR spectra are shown on this page with an. Benzene shows a less intense absorption in 230-276 nm range. Benzene shows a less intense absorption in 230-276 nm range.
 Source: orgchemboulder.com
Source: orgchemboulder.com
Benzene shows a less intense absorption in 230-276 nm range. From IR spectra IR spectra provide valuable informa on about local configura ons of atoms in molecules. Some of the characteristic absorptions are mνcm-1mllVibration mode. Around 1200-1300 cm-1. Beauchamp Spectroscopy Tables 4 Zclassesspectroscopyall spectra tables for webDOC IR Flowchart to determine functional groups in a compound all values in cm-1.
Source: webbook.nist.gov
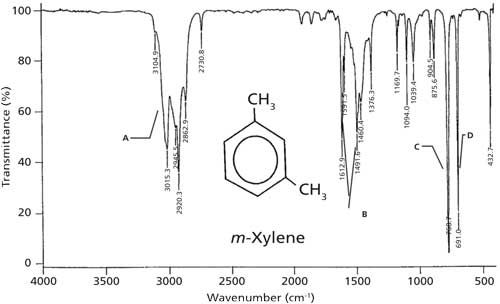
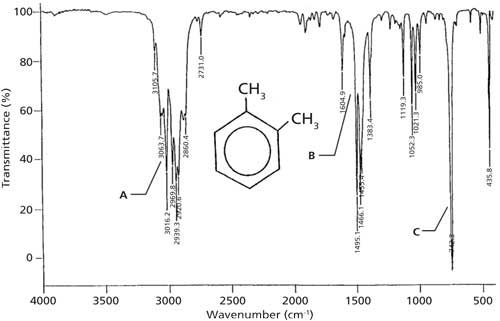
Is 1 2 4 Trisubstituted Benzene Active In The Range 680 720 Cm. 93 rows In physical and analytical chemistry infrared spectroscopy IR spectroscopy is a. The infrared spectra of deuterobenzenesulphonyl compounds may be useful to distinguish between the alternative assignments since the frequency shift on ring deuteration for the mixed vibration of the ring breathing and the C-S stretching modes must be much smaller than that for a C-H in-plane 3600 3200 2800 2400 1600 1400 1200 1100 1000 900 800 700 FREQUENCY cm. Ir Spectrum Table Benzene Ring Major Absorptions In Ir Spectra Of Bsmps Diffe Sizes See also Detroit Tigers Seat Chart Quantitative Comonomer Analysis Of Polyacrylates Via Ir Solved Predict The Ir Spectra Of Following Molecules Infrared Spectroscopy See also Time Warner Cable Arena Seating Chart For Concerts. The use of infrared spectroscopy for determining the substituent pattern of substituted benzene rings is illustrated by the following data and the spectra examples underneath.
 Source: spectroscopyonline.com
Source: spectroscopyonline.com
The use of infrared spectroscopy for determining the substituent pattern of substituted benzene rings is illustrated by the following data and the spectra examples underneath. Substitution sensitive bands of benzene below 1000 cm 1. Benzene shows a less intense absorption in 230-276 nm range. Continuing our theme of investigating the infrared spectra of hydrocarbons we look at the nature of aromatic bonding and why aromatic rings have unique structures bonding and infrared spectra. 93 rows In physical and analytical chemistry infrared spectroscopy IR spectroscopy is a.
 Source: spectroscopyonline.com
Source: spectroscopyonline.com
CH stretch from 3100-3000 cm -1 overtones weak from 2000-1665 cm -1 CC stretch in-ring from 1600-1585 cm -1 CC stretch in-ring from 1500-1400 cm -1 CH oop from 900-675 cm -1 The spectrum of toluene is shown below. The use of infrared spectroscopy for determining the substituent pattern of substituted benzene rings is illustrated by the following data and the spectra examples underneath. The full IR spectra are shown on this page with an. Alkyl substituents are Electron Donating Groups inductive effect lower the bond strength of CO ketone carbonyls have slightly lower stretching frequencies 1715 7 cm-1. Then we examine in detail the spectra of mono- and di-substituted benzene rings and learn that infrared spectroscopy easily distinguishes between ortho- meta- and para.
 Source: chem.libretexts.org
Source: chem.libretexts.org
See also Standard Window Curtain Rod Length. Pics of. Alkyl substituents are Electron Donating Groups inductive effect lower the bond strength of CO ketone carbonyls have slightly lower stretching frequencies 1715 7 cm-1. Carbonyl - compounds For simple aldehydes and ketones the stretching vibration of the carbonyl group has a strong infrared absorption between 1710 and 1740 cm-1. Benzene primarily absorbs through a be a π-π transition over the range from 160-208 nm wi th a λ max v alue of about 178 nm.
 Source: docbrown.info
Source: docbrown.info
Substitution sensitive bands of benzene below 1000 cm 1. Do Halogens Appear In The Ir Spectrum For Example A Molecule. How Could Ir Spectroscopy Be To Distinguish Between Benzene Infrared Spectral Interpretation Ir Spectra Of Anion Doped Polyanillines Table Ir Spectra Tricks For Indentifying The 5 Zones Infrared Spectrometry Example 7 Distinguishing Structural Isomers Mono And Disubstituted Benzene Structure And Thermal Properties Of Lignins Characterization By. 93 rows In physical and analytical chemistry infrared spectroscopy IR spectroscopy is a. Together creating precise yet practical optical solutions enabling you to solve problems.
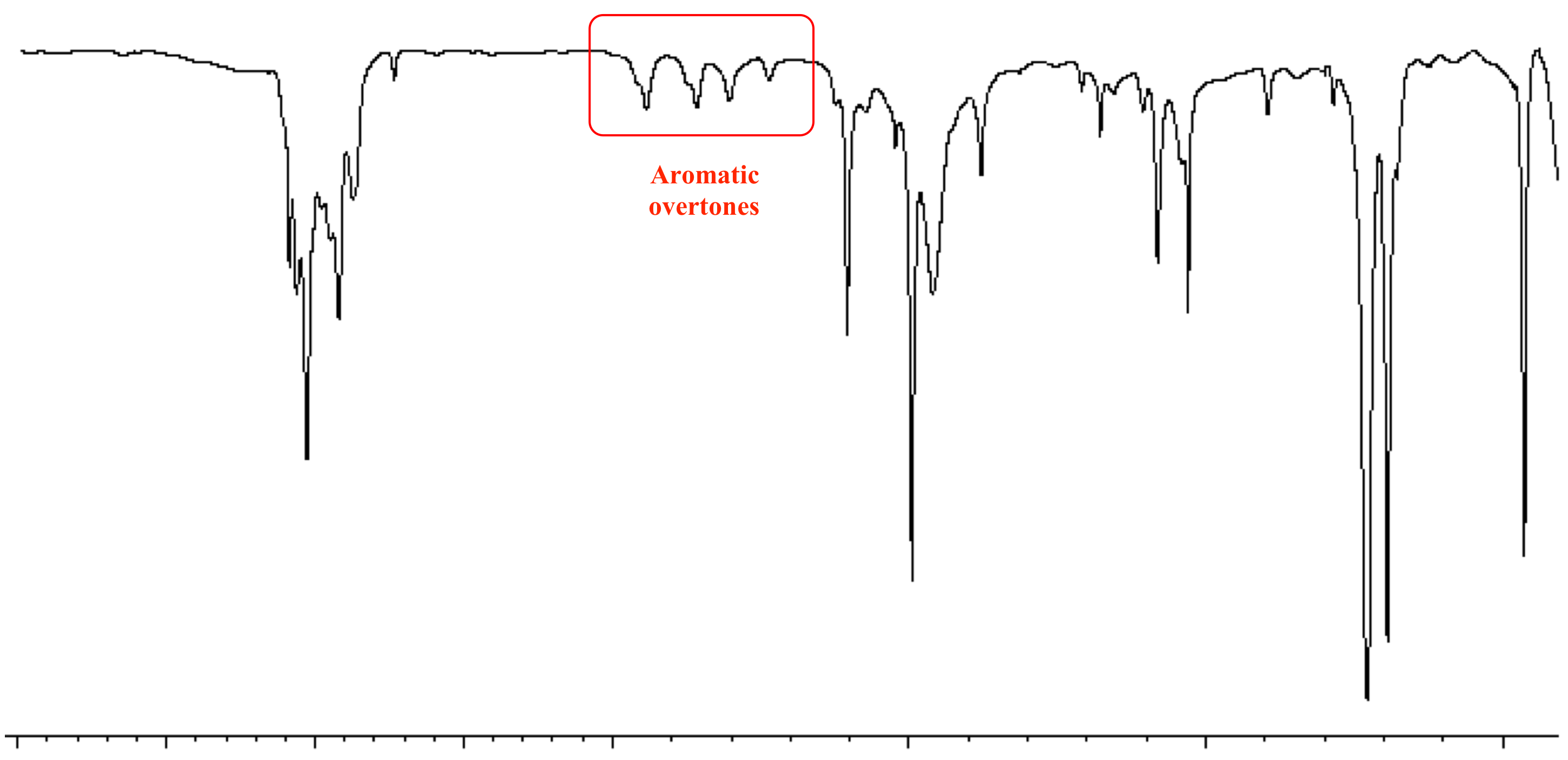 Source: chem.ucla.edu
Source: chem.ucla.edu
The use of infrared spectroscopy for determining the substituent pattern of substituted benzene rings is illustrated by the following data and the spectra examples underneath. The position of substitution on a benzene ring can sometimes be determined from the IR spectrum. SOLUTION 10 IN CCl4 FOR 3800-1300 10 IN CS2 FOR 1300-625 AND 10 IN CCl4 FOR 625-240 CM-1 VS. Has CO band 1650-1800 cm-1 very strong does not have CO band IR Spectrum aldehydes C O aldehyde C-H 1725-1740 saturated 1660-1700 unsaturated 2860-2800 2760-2700 both. IR Spectroscopy Part II 1.
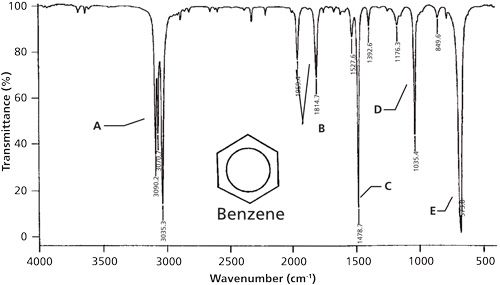 Source: spectroscopyonline.com
Source: spectroscopyonline.com
Benzene - SpectraBase John Wiley Sons Inc. Is 1 2 4 Trisubstituted Benzene Active In The Range 680 720 Cm. The normal way to approach interpretation of an IR spectrum is to examine the functional group region to determine which groups might be present then to. Alkyl substituents are Electron Donating Groups inductive effect lower the bond strength of CO ketone carbonyls have slightly lower stretching frequencies 1715 7 cm-1. The use of infrared spectroscopy for determining the substituent pattern of substituted benzene rings is illustrated by the following data and the spectra examples underneath.
 Source: docbrown.info
Source: docbrown.info
The three possible isomers are easy to differen ate by IR. Carbonyl - compounds For simple aldehydes and ketones the stretching vibration of the carbonyl group has a strong infrared absorption between 1710 and 1740 cm-1. Around 1200-1300 cm-1. 93 rows In physical and analytical chemistry infrared spectroscopy IR spectroscopy is a. Also benzene rings show overtones in the 1500-1700 cm-1region even though these arise from complex ring deformations.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title benzene ring ir spectrum by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






