Your Aromatic ring ir spectrum images are available in this site. Aromatic ring ir spectrum are a topic that is being searched for and liked by netizens now. You can Get the Aromatic ring ir spectrum files here. Get all free vectors.
If you’re searching for aromatic ring ir spectrum images information linked to the aromatic ring ir spectrum topic, you have come to the right site. Our website frequently gives you hints for seeing the highest quality video and image content, please kindly hunt and find more enlightening video articles and images that match your interests.
Aromatic Ring Ir Spectrum. CC Aromatic rings lS00-1600 Variable CC Alkynes 2100-2260 Variable CN Amines amides 1180-1360 Strong CN Nitriles 2210-2280 Strong CO Alcohols ethers carboxylic acids esters 1050-1300 Strong CO Aldehydes ketones carboxylic acids esters 1690-1760 Strong NO2 Nitro compounds 1500-1570 Strong 1300- 1370 Strong. The out-oi-plane C-H bending vibrations in the region 625-900 cm-1 M. Recall that benzene rings have. 40 Ca 13C NMR Spectroscopy of Aromatic Compounds As with other 13C NMR spectra aromatic compounds display single lines for each unique carbon environment in a benzene ring.
 Ftir Spectrum Of Uncured Epoxy Resin Peaks At I 3054 89 Cm 1 Download Scientific Diagram From researchgate.net
Ftir Spectrum Of Uncured Epoxy Resin Peaks At I 3054 89 Cm 1 Download Scientific Diagram From researchgate.net
Recall that benzene rings have. Phenol Home Infrared Spectrum of an Aromatic Alcohol. If the nitro group is attached to an aromatic ring the NO stretching bands shift to down to slightly lower wavenumbers. 4NCAT presents an H-bond between. 1597 cm-1 Skeletal vibrations carbon-carbon stretching within the ring. The IR spectrum plays an important role and provides valuable information related to the functional groups in pentacyclic triterpenoids.
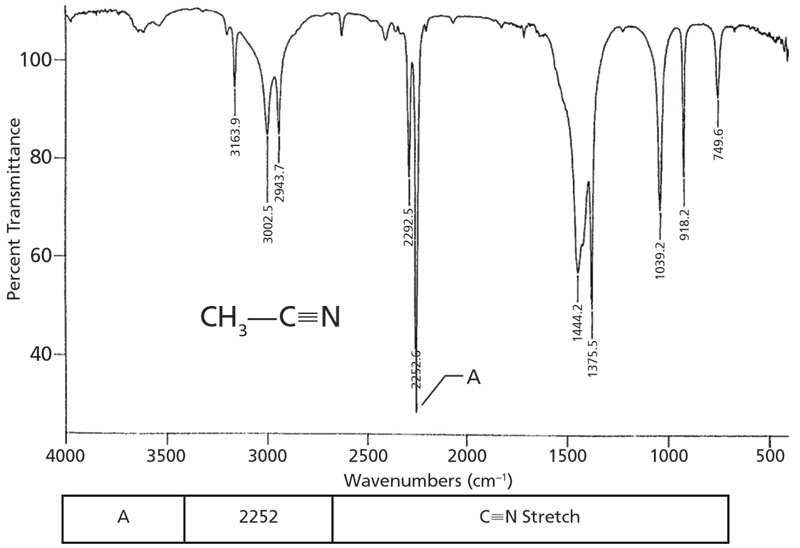
The NO stretching vibrations in nitroalkanes occur near 1550 cm -1 asymmetrical and 1365 cm -1 symmetrical the band at 1550 cm -1 being the stronger of the two.
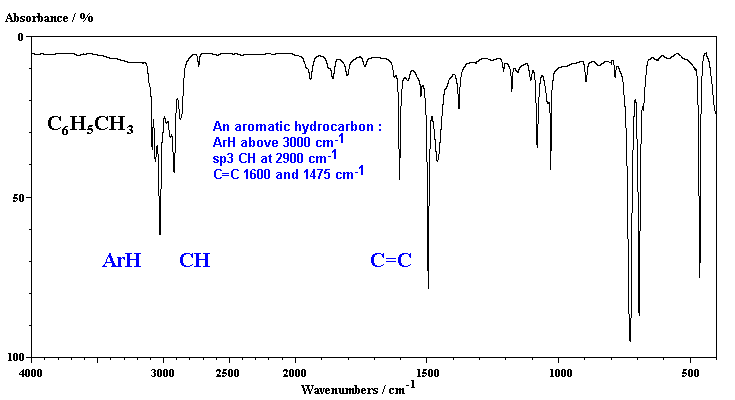
3550 - 3200 broad s See Free vs. FASSEL Institute for Atomic Research and Department of Chemistry Iowa State College Ames Iowa Received 15 July 1954 Acceptedor publication. INTRODUCTION 11 Aramide 1 Aromatic polyamide described under the generic term aramide a manufactured fiber in which the. 690 cm-1 Out of plane bend Aromatic Ring. However CC bonds also have C-H stretches in this region so we must look for other aromatic peaks to confirm their presence. CH stretch from 3100-3000 cm -1 overtones weak from 2000-1665 cm -1 CC stretch in-ring from 1600-1585 cm -1 CC stretch in-ring from 1500-1400 cm -1 CH oop from 900-675 cm -1 Note that this is at slightly higher frequency than is the CH stretch in alkanes.
Source: webbook.nist.gov
Zfilesclassesspectroscopytypical spectra chartsDOC Carbonyl Highlights stretching wave numbers C O R H Aldehydes Ketones Acids Amides Anhydrides Acid Chlorides saturated 1725 conjugated 1690 aromatic 1700 C O R R saturated 1715 conjugated 1680 aromatic 1690 6 atom ring 1715 5 atom ring 1745 4 atom ring 1780 3 atom ring. The out-oi-plane C-H bending vibrations in the region 625-900 cm-1 M. What is the IR range of an aromatic ring. FASSEL Institute for Atomic Research and Department of Chemistry Iowa State College Ames Iowa Received 15 July 1954 Acceptedor publication. Group Wavenumbers And An Introduction To The Spectroscopy Of Benzene Rings.
 Source: researchgate.net
Source: researchgate.net
Latter ones are usually weak or medium in intensity. 4NCAT presents an H-bond between. There are three ways of arranging two substituents around a benzene ring meaning that disubstituted rings have three structural isomers hence the need for a discussion of structural isomers and substituted. Compounds in which the carbonyl group is conjugated with aromatic rings double bonds or triple bonds have lower carbonyl stretching frequencies than unconjugated carbonyl com-pounds. In physical and analytical chemistry infrared spectroscopy IR spectroscopy is a technique used to identify chemical compounds based on the way infrared radiation is absorbed by the compound.
 Source: docbrown.info
Source: docbrown.info
Ir Spectrum Table Aromatic Ring. Recall that benzene rings have. There are three ways of arranging two substituents around a benzene ring meaning that disubstituted rings have three structural isomers hence the need for a discussion of structural isomers and substituted. CC Aromatic rings lS00-1600 Variable CC Alkynes 2100-2260 Variable CN Amines amides 1180-1360 Strong CN Nitriles 2210-2280 Strong CO Alcohols ethers carboxylic acids esters 1050-1300 Strong CO Aldehydes ketones carboxylic acids esters 1690-1760 Strong NO2 Nitro compounds 1500-1570 Strong 1300- 1370 Strong. IR Spectroscopy The principal infrared absorption of aldehydes and ketones is the CAO stretching absorption a strong absorption.
 Source: chem.ucla.edu
Source: chem.ucla.edu
IR Spectroscopy Part II 1. 690 cm-1 Out of plane bend Aromatic Ring. About 3375 cm-1 O-H stretch. 3049 cm-1 Antisymmetric and symmetric C-H stretch Aromatic Ring. There are three ways of arranging two substituents around a benzene ring meaning that disubstituted rings have three structural isomers hence the need for a discussion of structural isomers and substituted.
 Source: docbrown.info
Source: docbrown.info
Carboxylic Acid O-H Stretch. Recall that aromatic rings are unsaturated and hence have C-H stretching peaks between 3100 and 3000 9. Recall that benzene rings have. In aromatic compounds each band in the spectrum can be assigned. What is the IR range of an aromatic ring.
 Source: orgchemboulder.com
Source: orgchemboulder.com
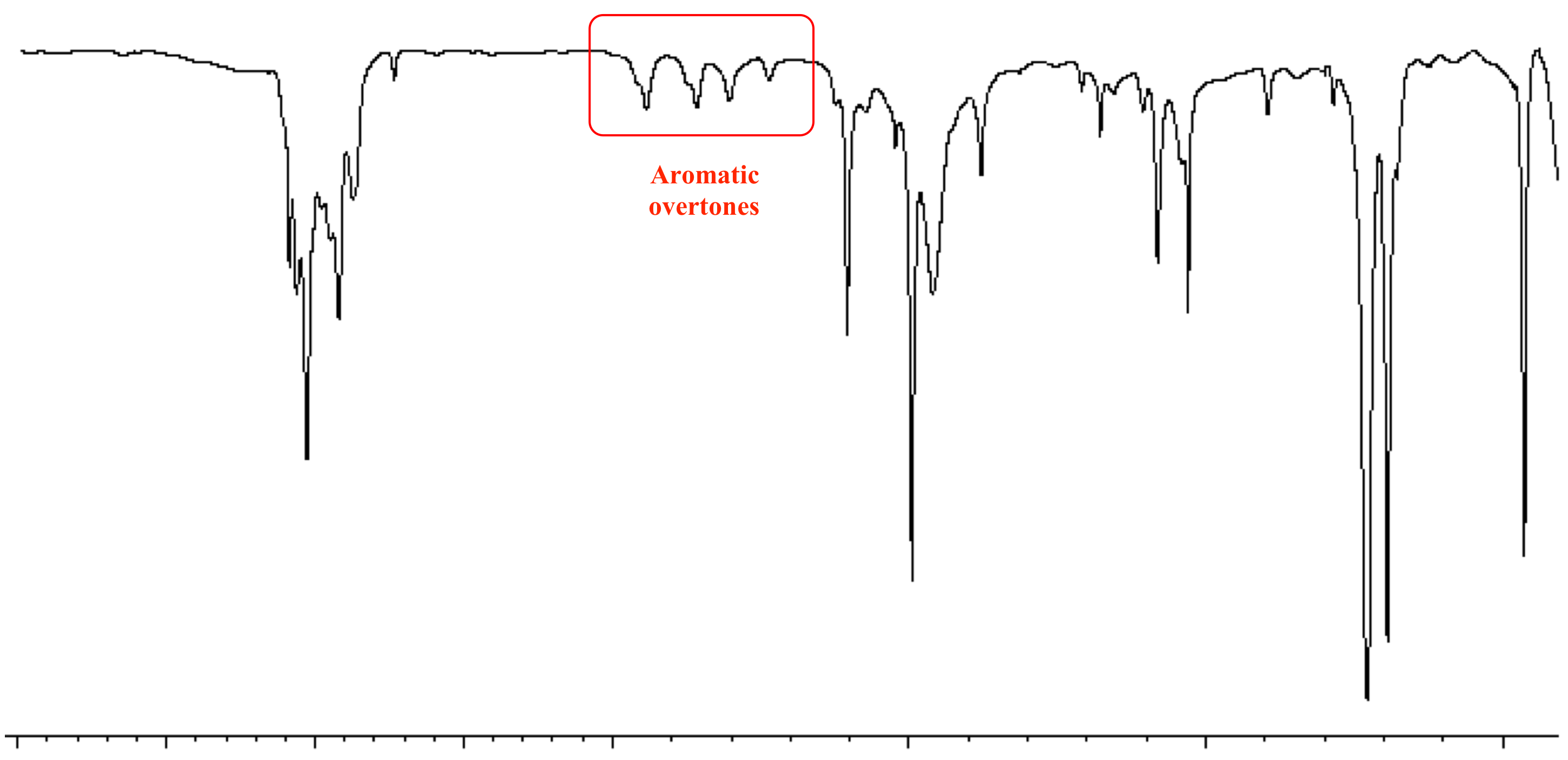
IR Spectroscopy Part II 1. Recall that benzene rings have. IR spectra of triterpenoids have two characteristically regions. Compounds in which the carbonyl group is conjugated with aromatic rings double bonds or triple bonds have lower carbonyl stretching frequencies than unconjugated carbonyl com-pounds. 2000-1700 cm-1 Aromatic Overtone.
 Source: chem.ucalgary.ca
Source: chem.ucalgary.ca
CH stretch from 3100-3000 cm -1 overtones weak from 2000-1665 cm -1 CC stretch in-ring from 1600-1585 cm -1 CC stretch in-ring from 1500-1400 cm -1 CH oop from 900-675 cm -1 Note that this is at slightly higher frequency than is the CH stretch in alkanes. The absorptions in this range do not apply only to bonds in organic molecules. The range from 2850-3000 cm-1belongs to saturated systems alkanes sp3 example 1 while the peaks from 3000-3100 cm-1indicate an unsaturated system alkenes sp2example 2. Strong aromatic absorptions between 1650 cm-1 and 1450 cm-1 Strong oop absorptions at 725 cm-1 and 694 cm-1 monosubstituted phenyl If IR spectroscopy is not conclusive you can add NMR spectroscopy. CC Aromatic rings lS00-1600 Variable CC Alkynes 2100-2260 Variable CN Amines amides 1180-1360 Strong CN Nitriles 2210-2280 Strong CO Alcohols ethers carboxylic acids esters 1050-1300 Strong CO Aldehydes ketones carboxylic acids esters 1690-1760 Strong NO2 Nitro compounds 1500-1570 Strong 1300- 1370 Strong.
 Source: dummies.com
Source: dummies.com
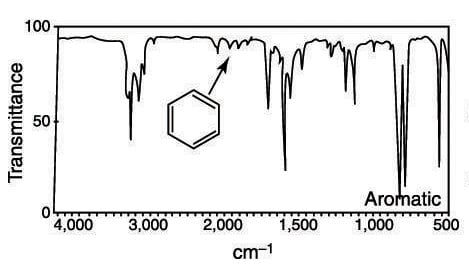
Latter ones are usually weak or medium in intensity. Aromatics show a lot more bands in an IR spectrum than do alkanes alkenes and alkynes. Characteristic IR Absorption Frequencies. 4NCAT presents an H-bond between. Alkyl groups show up to the right of 3000 aromatic CH stretches to the left of 3000.
 Source: spectroscopyonline.com
Source: spectroscopyonline.com
Latter ones are usually weak or medium in intensity. IR Spectroscopy Part II 1. In physical and analytical chemistry infrared spectroscopy IR spectroscopy is a technique used to identify chemical compounds based on the way infrared radiation is absorbed by the compound. While Aromatic and Alkene C-H stretches both occur just over 3000 the CC aromatic stretches appear between 1600 and 1450 outside the usual range for alkenes which is near 1650. The out-oi-plane C-H bending vibrations in the region 625-900 cm-1 M.
 Source: researchgate.net
Source: researchgate.net
Aromatic IR spectra are messy and difficult. Pergamon Press Ltd London The infrared spectra of aromatic compounds I. Aramide p-phenylene terphthalamide m-phenylene terphthalamide FT-IR spectroscopy ATR I. What is the IR range of an aromatic ring. 3550 - 3200 broad s See Free vs.
 Source: spectroscopyonline.com
Source: spectroscopyonline.com
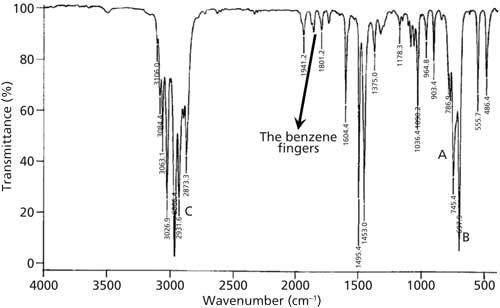
Infrared Spectroscopy Quantitative Comonomer Analysis Of Polyacrylates Via Ir Infrared Spectra Identifying Functional Groups See also Graco Affix Backless Booster Seat Canadian Tire. CC Aromatic rings lS00-1600 Variable CC Alkynes 2100-2260 Variable CN Amines amides 1180-1360 Strong CN Nitriles 2210-2280 Strong CO Alcohols ethers carboxylic acids esters 1050-1300 Strong CO Aldehydes ketones carboxylic acids esters 1690-1760 Strong NO2 Nitro compounds 1500-1570 Strong 1300- 1370 Strong. 40 Ca 13C NMR Spectroscopy of Aromatic Compounds As with other 13C NMR spectra aromatic compounds display single lines for each unique carbon environment in a benzene ring. Alkyl groups show up to the right of 3000 aromatic CH stretches to the left of 3000. There are several peaks in this region in Figure i confirming the presence of unsaturated rings.
 Source: docbrown.info
Source: docbrown.info
IR spectra of triterpenoids have two characteristically regions. IR Spectroscopy Part II 1. Recall that aromatic rings are unsaturated and hence have C-H stretching peaks between 3100 and 3000 9. Aromatic IR spectra are messy and difficult. Carboxylic Acid O-H Stretch.
 Source: orgchemboulder.com
Source: orgchemboulder.com
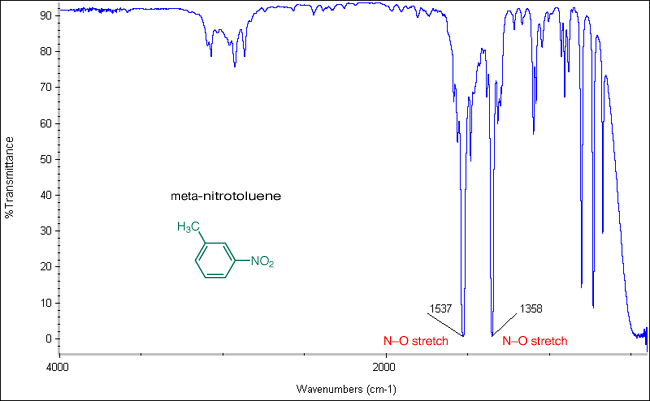
The NO stretching vibrations in nitroalkanes occur near 1550 cm -1 asymmetrical and 1365 cm -1 symmetrical the band at 1550 cm -1 being the stronger of the two. The range from 2850-3000 cm-1belongs to saturated systems alkanes sp3 example 1 while the peaks from 3000-3100 cm-1indicate an unsaturated system alkenes sp2example 2. The absorptions in this range do not apply only to bonds in organic molecules. About 3375 cm-1 O-H stretch. Recall that benzene rings have.
Source: webbook.nist.gov
FT-IR spectroscopy coupled with ATR technique is useful method to identify such polymers. Aromatic ring example 34. Alkyl substituents are Electron Donating Groups inductive effect lower the bond strength of CO ketone carbonyls have slightly lower stretching frequencies 1715 7 cm-1. However CC bonds also have C-H stretches in this region so we must look for other aromatic peaks to confirm their presence. Infrared spectra clearly show the presence of the H-bonds between OH vibrations being marked with and and NO 2 group substituents attached to the aromatic ring in the nitrocatechols as follows.
 Source: researchgate.net
Source: researchgate.net
If the nitro group is attached to an aromatic ring the NO stretching bands shift to down to slightly lower wavenumbers. One of the most telling bands is the presence of a band just to the left of 3000. However CC bonds also have C-H stretches in this region so we must look for other aromatic peaks to confirm their presence. 3500 - 3300 m Primary amines produce. 3000 - 2500 broad v Amine N-H Stretch.
 Source: spectroscopyonline.com
Source: spectroscopyonline.com
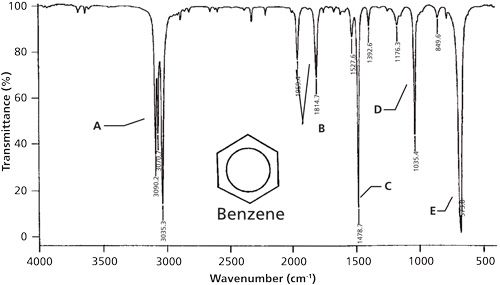
Alkyl groups show up to the right of 3000 aromatic CH stretches to the left of 3000. 4NCAT presents an H-bond between. 1H NMR spectroscopy Hydrogens directly attached to an aromatic ring appear at about 7 ppm - 9 ppm as in the 1H NMR spectrum of toluene. Aromatic C-H Stretch Aromatic C-H Bending Aromatic CC Bending 3030 v 860 - 680 s 1700 - 1500 mm AlcoholPhenol O-H Stretch. Characteristic IR Absorption Frequencies.
 Source: docbrown.info
Source: docbrown.info
Zfilesclassesspectroscopytypical spectra chartsDOC Carbonyl Highlights stretching wave numbers C O R H Aldehydes Ketones Acids Amides Anhydrides Acid Chlorides saturated 1725 conjugated 1690 aromatic 1700 C O R R saturated 1715 conjugated 1680 aromatic 1690 6 atom ring 1715 5 atom ring 1745 4 atom ring 1780 3 atom ring. Infrared Spectroscopy Quantitative Comonomer Analysis Of Polyacrylates Via Ir Infrared Spectra Identifying Functional Groups See also Graco Affix Backless Booster Seat Canadian Tire. The 13C NMR spectra of bromobenzene and p-bromoethylbenzene are shown below for comparisonThere are four different carbon. Assignment Of Major Absorption Ir Spectra Peaks In Wood Forming Tissue Table. The IR spectrum plays an important role and provides valuable information related to the functional groups in pentacyclic triterpenoids.
 Source: researchgate.net
Source: researchgate.net
Phenol Home Infrared Spectrum of an Aromatic Alcohol. Spectrochlmica Acta 1955 Vol. Strong aromatic absorptions between 1650 cm-1 and 1450 cm-1 Strong oop absorptions at 725 cm-1 and 694 cm-1 monosubstituted phenyl If IR spectroscopy is not conclusive you can add NMR spectroscopy. 3550 - 3200 broad s See Free vs. The compounds 3NCAT and 5M3NCAT exhibit an H-bond between the OH and one of the O atoms from the NO 2 vicinal group.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title aromatic ring ir spectrum by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






